- Advanced BSL3 Work Practices and Procedures
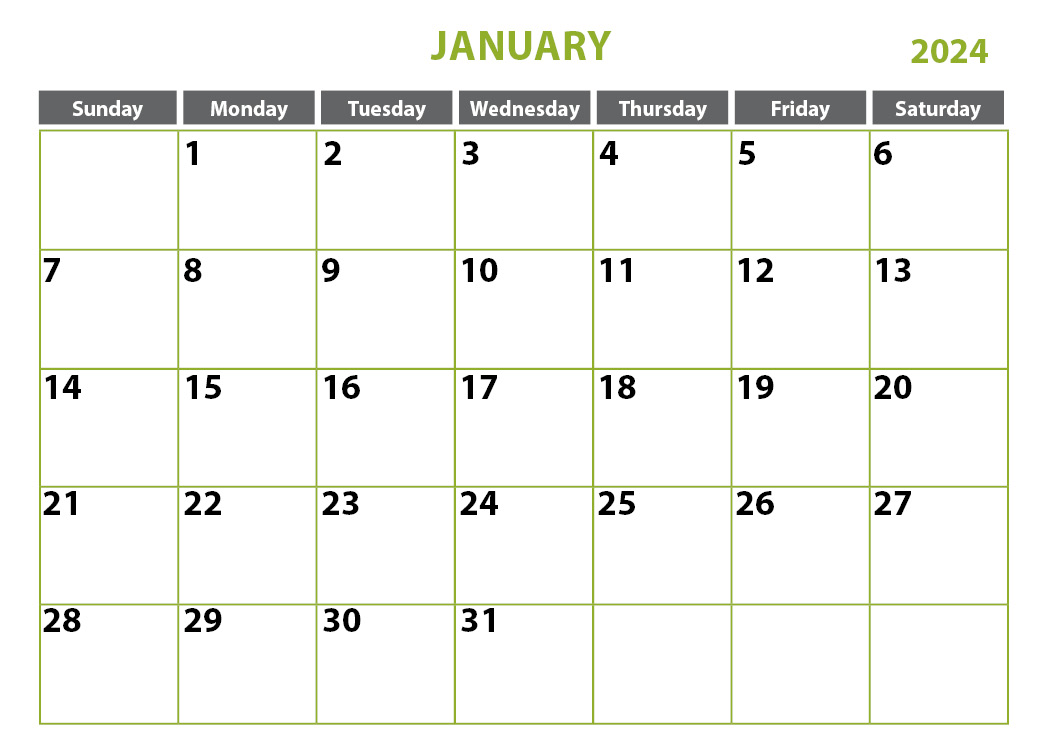
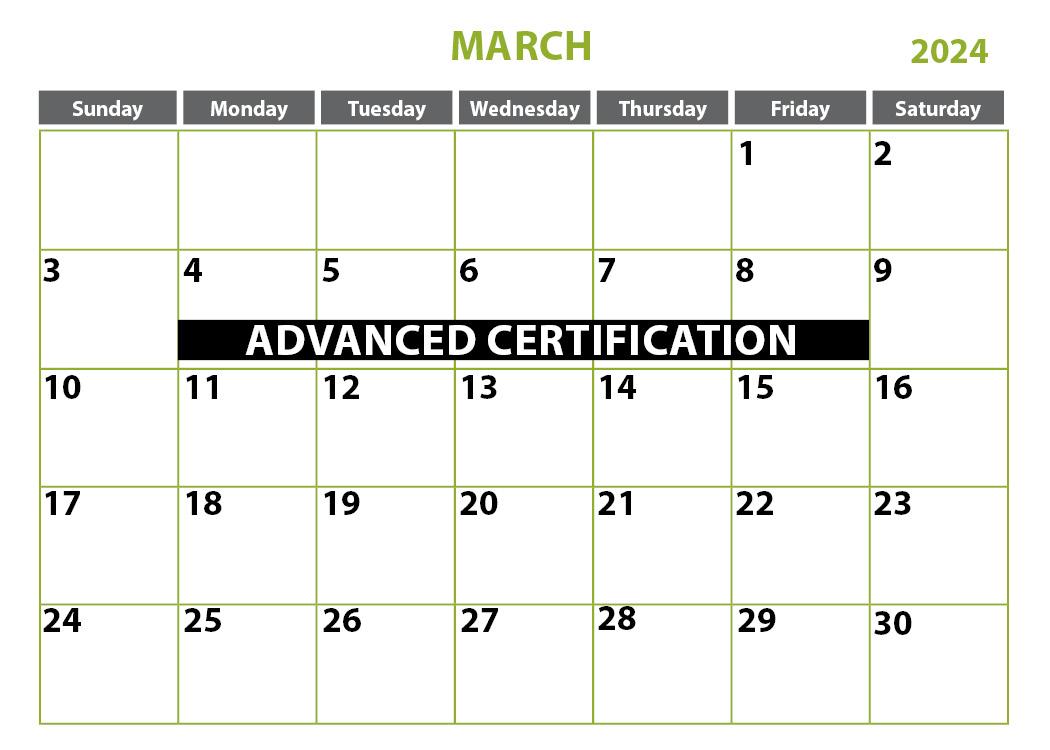
- Advanced Certification
- BSL3 Facilities: Design and Operation
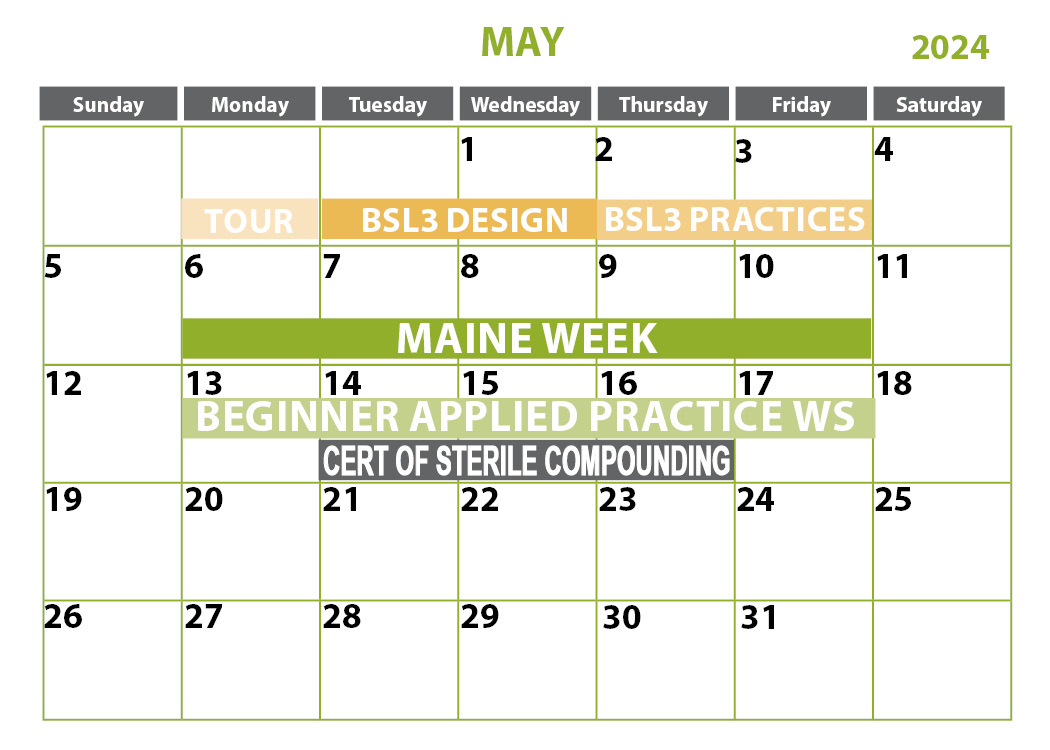
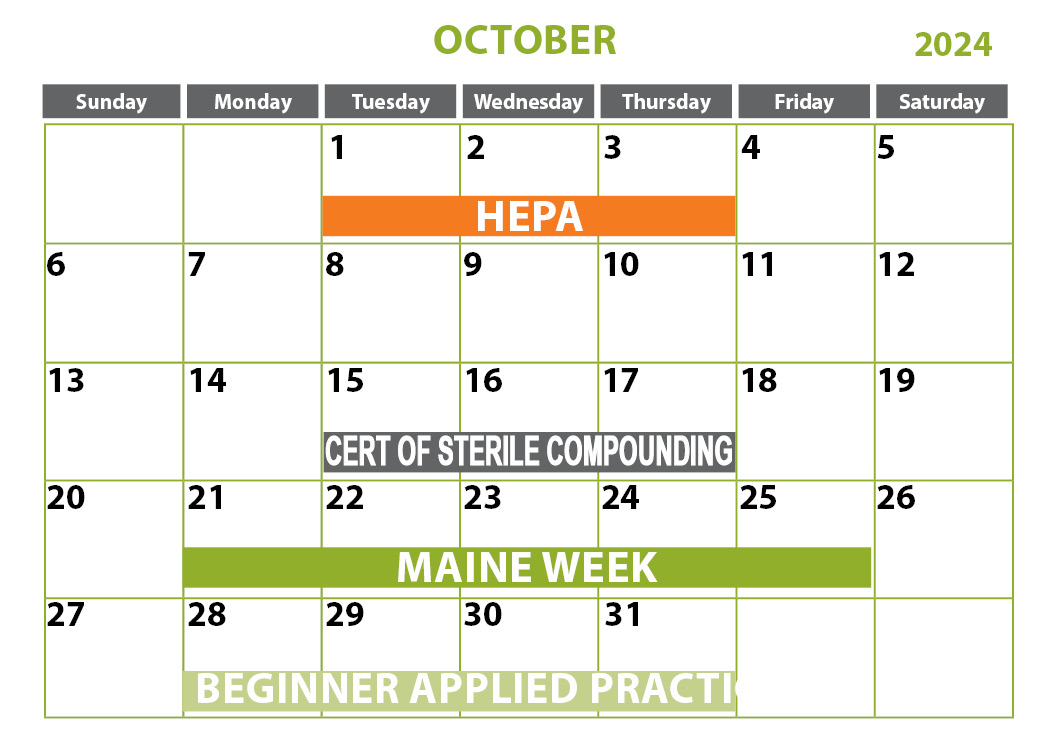
- Certification of Sterile Compounding Facilities
- HVAC Systems and Laboratory Design
- Safety Cabinet Technology
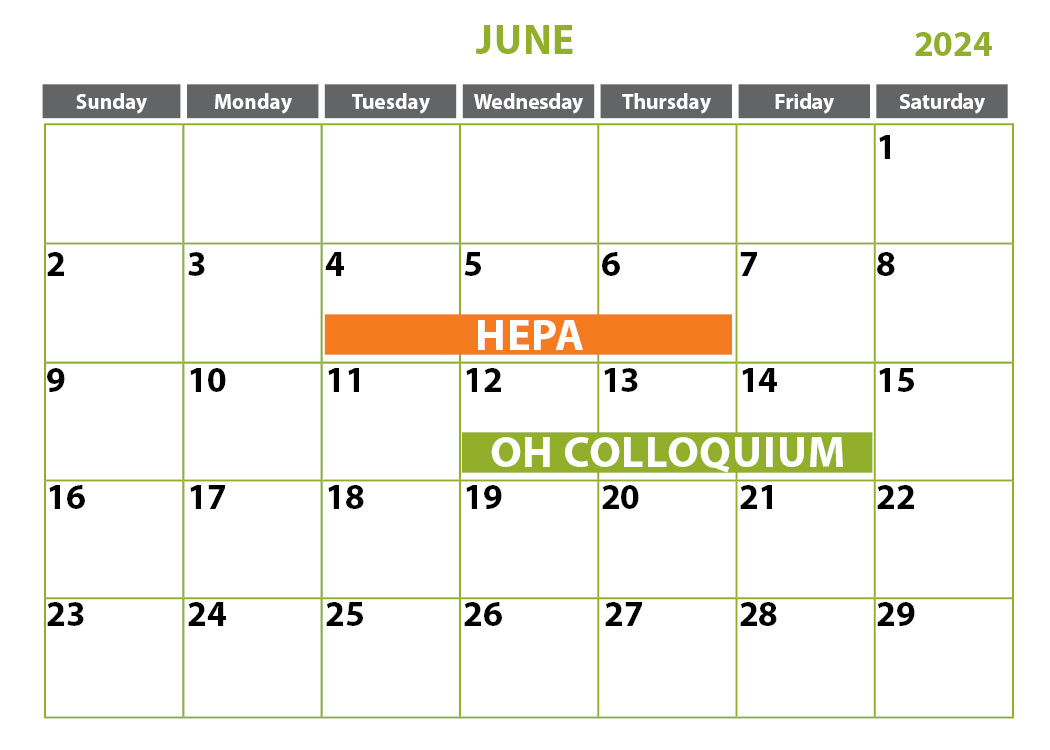
- Testing of HEPA Filtered Systems and Pharmaceutical Cleanrooms
- Verifying BSL3 Facility Performance
Certification of Sterile Compounding Facilities
October 15-17, 2024 | St. Louis, MO
This three-day class uses lecture, hands-on workshops and group activities to provide a review of the facility design, and environmental control requirements of USP Chapter <797> and the NIOSH Alert. The “Engineering Control Performance Verification” section of USP <797> is covered in detail. The specific certification procedures outlined in the Controlled Environment Testing Association’s (CETA) CAG-003 are also discussed and utilized for the course’s hands-on workshops. In addition, CAG -001-2005 and CAG-002-2006 will be examined and utilized for the certification of compounding aseptic isolators.
Who Is This Course For?
Certifiers, Cleanroom Designers, Cleanroom Contractors and anyone designing or building cleanrooms for sterile compounding.
Course Instructors
The lead instructor for this course is Jim Wagner, Principal, Controlled Environment Consulting.
Other instructors include:
- Erin Thane, Senior Director of Lab Services- PA, Azzur Labs
- Mark St. Marie, Director of Business Development, Eurofins Built Environment Testing USA Analytics
- Jeff Smith, Sr. Manager, Training & Technical Svcs., Controlled Environment Services Inc.| Life Sciences, A Subsidiary of STERIS Corporation
Course Tuition
Tuition of $2495 must be paid in full to guarantee a space in the class. Tuition includes: course manual, lunch each day, an Eagleson Institute certificate and a special class reception and dinner with plenty of time to network with peers and instructors.