- Advanced BSL3 Work Practices and Procedures
- Advanced Certification
- BSL3 Facilities: Design and Operation
- Certification of Sterile Compounding Facilities
- HVAC Systems and Laboratory Design
- Safety Cabinet Technology
- Testing of HEPA Filtered Systems and Pharmaceutical Cleanrooms
- Verifying BSL3 Facility Performance
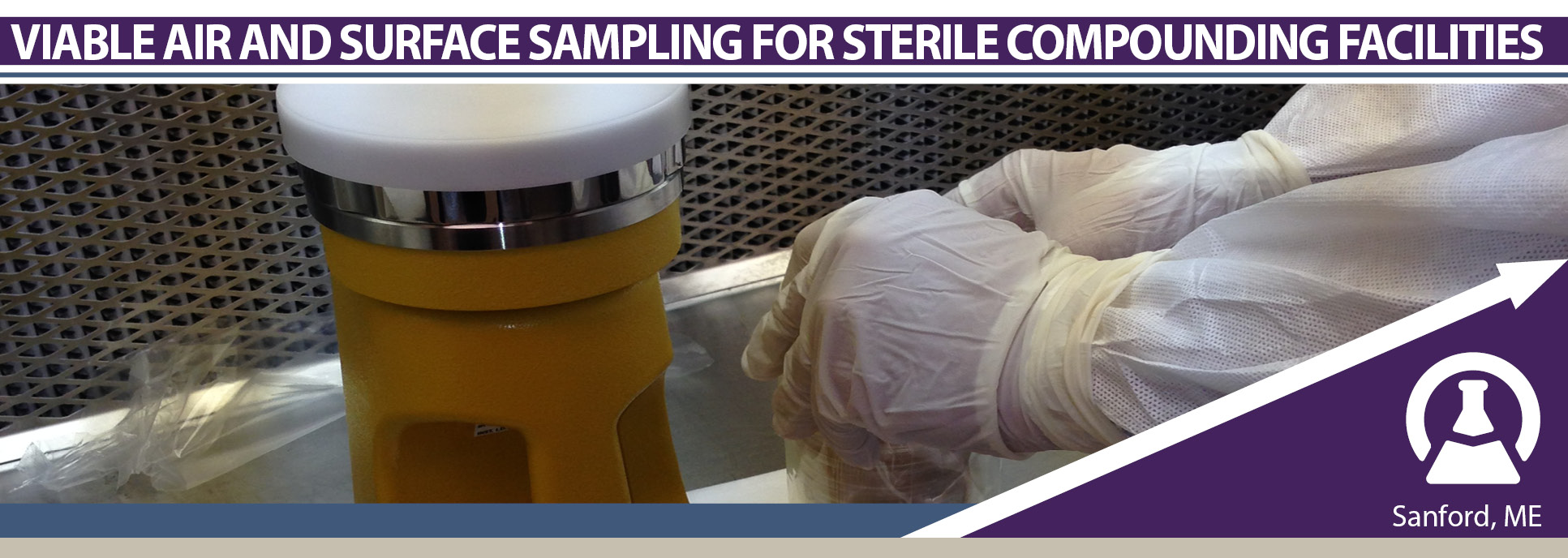
Viable Air and Surface Sampling for Sterile Compounding Facilities
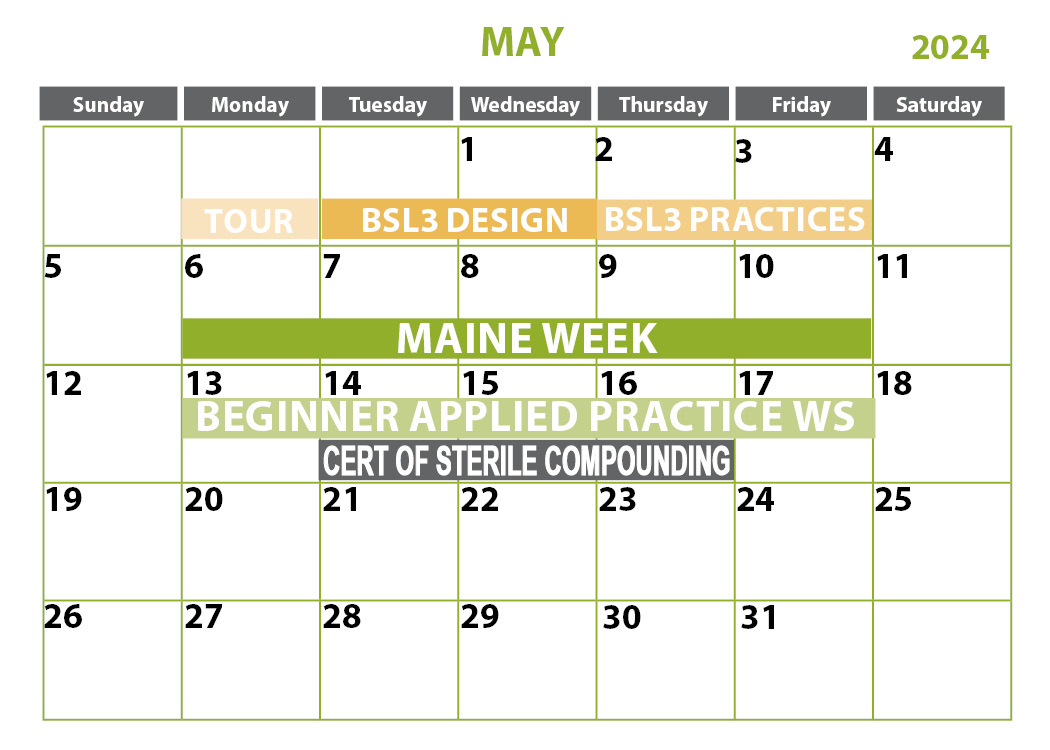
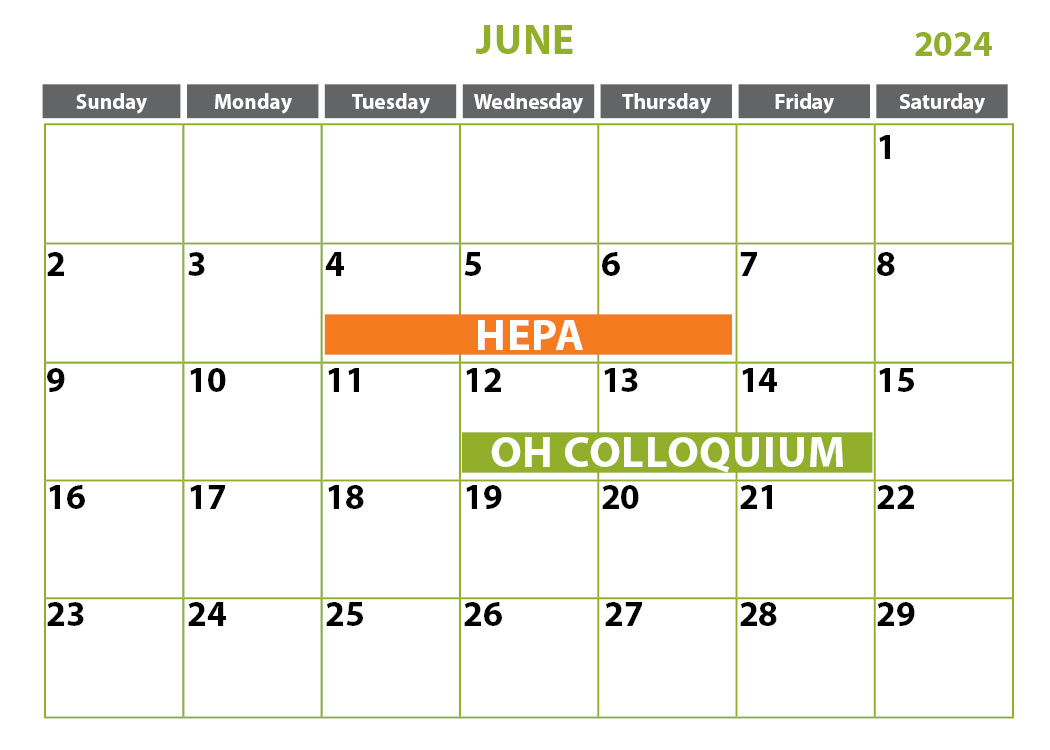
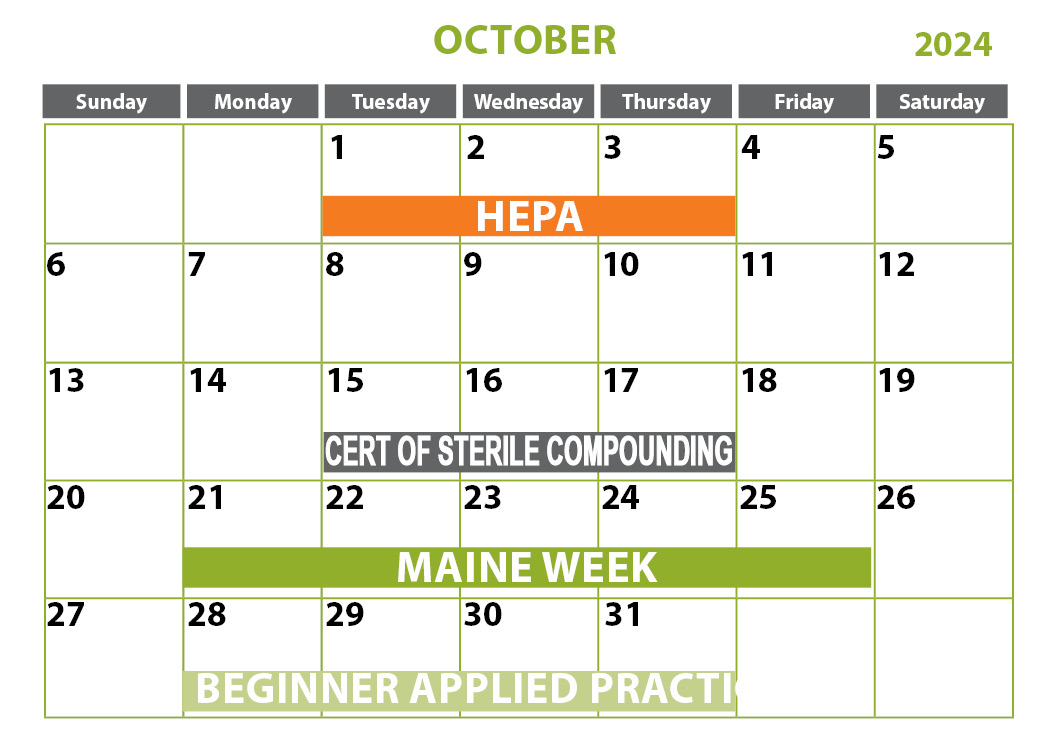
April 2-4, 2024 | Sanford, ME
***April 4, 2024 is optional half-day competency only
Viable Air and Surface Sampling for Sterile Compounding Facilities is a two-day training, with the option of a half-day competency assessment, utilizing both lecture and hands-on activities. The class addresses everything a certification company must consider when developing robust viable sampling procedures for servicing their USP <797> customers, as well as, how to support the customer through viable excursion investigations. As part of the competency assessment, attendees will be evaluated on their sampling technique, ability to choose appropriate sample locations, and knowledge of conducting a sampling session.
Learning Objectives
Sampling and Contamination Control Overview
- Discuss the general requirements of a USP <797> compliant viable sampling program.
- Explain the value and limitations of a viable sampling program.
- List other industry standards and guidance documents that aid in the development of an effective program.
- Identify suitable media based on sampling needs.
- Discuss why achieving a microbial state of control is the overall goal of a sterile compounding facility.
- Explain how cleaning and disinfection is a fundamental part of a contamination control program.
The Certification Company’s Sampling Program
- Discuss the required and suggested best practice components of a viable sampling program and apply them to the service delivered to customers.
- List the necessary standard operating procedures (SOPs) and forms used to document the sampling process.
- Identify valuable sampling locations based on sterile compounding operations and workflow, which will assist you customer in making data driven decisions.
- Summarize what trending data is, why it is important to the program, and how different techniques can be implemented.
- List the elements of a robust viable sampling training program.
Skill Building: Choosing Sampling Locations and Creating Forms
- Choose USP <797> compliant and best practice sample locations.
- Create viable sampling data collection forms that include essential reporting elements.
Sampling Technique
- Recognize sampling equipment that meets the needs of a sterile compounding pharmacy.
- Recall the steps for collecting viable air and surface samples.
- Describe the proper technique for opening, manipulating, using, and repackaging media.
Skill Building: Viable Air and Surface Sampling Technique
- Properly load and unload a viable air sampler.
- Collect surface samples without inadvertently contaminating the sample.
Performing a Sampling Session
- Identify the necessary equipment, materials, and documentation that must be gathered to execute a viable sampling session.
- Describe the best sampling order to maintain the integrity of the samples and environment.
- Explain how to prepare samples for laboratory submission.
Skill Building: Sampling Practice Session
- Perform a viable sampling session, including gathering equipment and materials and collecting air and surface samples.
- Demonstrate the procedure to prepare samples for lab submission.
Incubation and Analysis
- Explain which incubation times and temperatures are conducive to the growth of cleanroom microorganisms.
- Describe the best method for counting microbial growth recovered on viable samples.
- List different techniques used to identify microorganisms.
How to Choose a Lab
- Evaluate a contract microbiology lab based on its ability to provide the necessary testing.
- Engage confidently in discussion with the lab.
- Identify the ideal characteristics of lab report.
Skill Building: Analyzing Lab Reports
- Identify missing information on lab reports.
- Interpret laboratory results to determine if they are acceptable and identify next steps in the event there is an exceeded action level.
Viable Sampling Excursions
- Evaluate viable sampling results to identify the best way to address microbial excursions.
- Describe the impact the recovery of certain microorganisms could have on a facility.
- List possible corrective and preventive actions relevant to remediating exceeded levels.
Competency Testing – Participants perform specific sampling-related skills and are assessed by the instructor. As part of the competency, participants are required to aseptically load and unload a viable air sampler inside a primary engineering control to demonstrate the ability to manipulate a sample without inadvertent contamination. Competency samples are submitted to a third-party microbiology contract testing laboratory for incubation and analysis. In addition, participants are required to identify sampling locations for a 3-room cleanroom suite and note rationale for why the location was chosen. Competency is demonstrated if the sample plate does not yield any microbial growth and if the sampling plan provides appropriate data to demonstrate a state of microbial control. Competency results are provided to the attendee.
Who Is This Course For?
Certifiers responsible for overseeing viable sampling activities within their organization, including program development, choosing sample locations, and training personnel.
Course Instructors
Abby Roth, founder of Pure Microbiology